Electric vehicles promise to help us move away from fossil fuels, but they present a new problem: how to get enough lithium that EV batteries require (SN: 5/7/19).
Materials scientist Chong Liu of the University of Chicago has some ideas. Existing technology can only extract lithium from sources with highly concentrated ions, such as solid rock or underground deposits of salty water called brine. Not only will these resources not be sufficient to meet the demand, but their extraction also brings environmental consequences (SN: 15.3.22).
Liu, however, has identified a material that could make extraction from untapped resources possible. It is looking at brines left over from geothermal and desalination processes, wastewater from fracking or even seawater, which could one day provide a large supply of lithium – if it can be tapped. A major challenge, however, is that seawater has a sodium-to-lithium ratio of approximately 20,000-to-1, nowhere near current sources that have ratios in the hundreds-to-1 range.
Extracting lithium ions from low-concentration solutions for commercial use will require much more research, Liu says. But her efforts have made great strides toward more efficient resource extraction, says University of Chicago molecular engineer Matthew Tirrell, who brought Liu on as an assistant professor in 2018. And it’s not just lithium extraction: “The work of “It’s setting the stage for other processes involving ions moving in porous, confined spaces,” he says, with future applications ranging from finding ways to speed up chemical reactions to potentially treating patients with mercury poisoning. or lead.
Selection for lithium
The method Liu is working with – called electrochemical intercalation – has only recently been used for resource extraction. First, the researchers immersed a material full of ion-sized pathways in salt water. The lithium ions in the water enter the channel network of the material and can be trapped there. But salt water also contains sodium ions, among other things, which push their way into those channels, reducing the amount of lithium that can be taken up.
Liu began hunting for an ideal suffocation material around 2016 during her postdoctoral work at Stanford University. She knew it was important to get it right: “The nature of this material will determine how selectivity [for lithium] we can get and what water source we can use,” says Liu. The better the selectivity, the more lithium is captured. So she scoured the scientific literature for a material that fit the bill. It had to be stable in water and maintain its structure even when filled with ions.
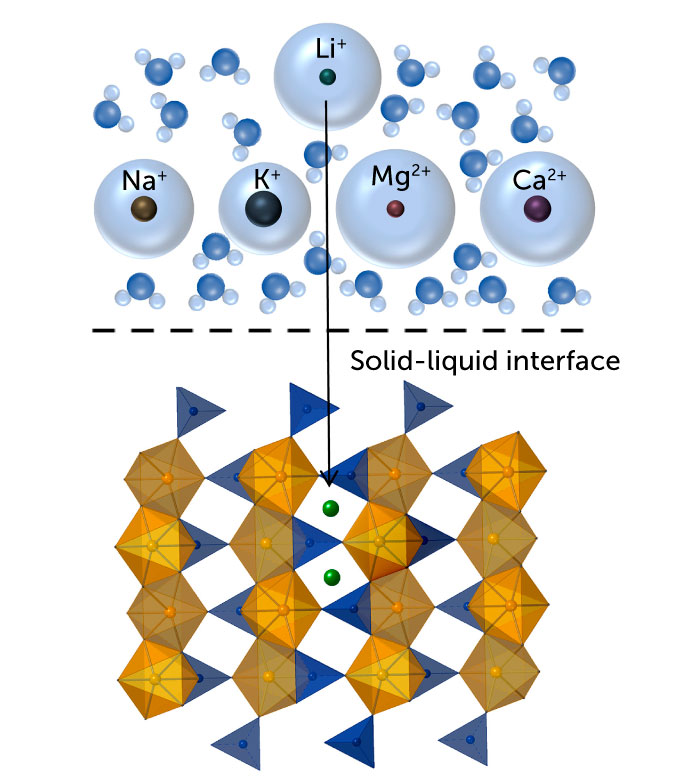
She eventually settled on iron phosphate. The oxygen in iron phosphate binds more readily to lithium than to competing sodium ions. Larger sodium ions can widen the channels, but the lithium-oxygen bonds keep the channels small and receptive to more lithium. Once the material is full of ions, it is moved to fresh water where researchers apply electric currents to expel the ions. Then they add a hydroxide, which combines with the lithium to form solid lithium hydroxide, the raw material used in EV batteries.
The amount of lithium that iron phosphate can currently extract is “spectacular,” says Stanford University physicist Steven Chu. Chu, the former US Secretary of Energy, worked with Liu during her postdoctoral studies. “By doing that, you can rest on your laurels and say, ‘This is great.’ But it is also directed [to ask]’Will it be practical?'”
In her lab in Chicago, Liu has been trying to make the material more efficient. Her team studies how lithium and sodium ions enter the material’s pores, in what order, and how they interact within the material. By better understanding the behavior of the ions, Liu says, the team can improve the material’s performance.
Mining to understand
Liu’s team is also developing a new method for separating rare earth elements, which she says is “actually more difficult” than extracting lithium. These 17 elements are essential to modern technologies, including wind turbines and smartphones (SN: 16.1.23). But often found together, they must be separated – a difficult task due to their similar size and composition.
Apart from these two methods, most of the laboratory’s work is focused on basic materials science. “We just accumulate more and more knowledge, so then we can start predicting things,” she says. That’s how it landed on iron phosphate: After much research, it was the first material the team tried.
It’s this deep understanding that makes Liu stand out, Chu says. There are “a small number of people you want to pay attention to,” because they often come up with smart, new approaches, he says, “and she’s one of them.”
Liu didn’t always dream of being a materials scientist, or even a professor. But during her Ph.D. at Stanford, she found she loved the research environment. “I really enjoy solving the puzzle,” she says. “This made me decide that maybe I want to do this job for the rest of my life. It’s fun.”
On weekends, she has a different kind of fun: playing Lego or riding bikes with her kids. She has started teaching her son about the solar system and photosynthesis. “We’re trying to see if we can just keep the fun going,” she says, “but get some of the science out.”
#materials #scientist #seeks #extract #lithium #untapped #resources
Image Source : www.sciencenews.org